Focused on different pharmacological aspects of tumor responses to new antitumor drugs. Our specific expertise is concentrated on molecular and cellular biology, in vitro/vivo tests on our murine and human xenografts models:
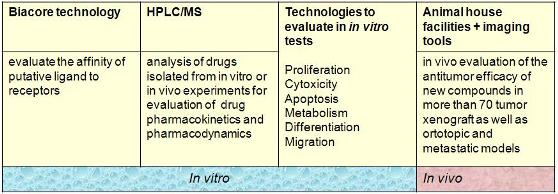
More than 70 xenograft plus orthotopic and metastatic models are available for the evaluation of new compound efficacy.
All experimental activities are under quality control with GLP - like standard procedures
Drugs screening by tailoring the models to their mechanism of action. Evaluation of antitumor activity of selected agents/rational combinations, in human solid tumor models characterized in terms of expression of relevant targets.
In Vitro Capabilities
CAREBIOS in vitro facilities are established for medium throughput multi - compound screening in over 200 human and mouse cell lines. We validate our in vitro research and development assays using reference compounds.
Cell proliferation and viability assays:
SRB fluorometric assay Tetrazolium uptake Lactate dehydrogenase (LDH)
Standard assays are available to evaluate:
- Migration
- Differentiation
- Cell Cycle
- Apoptosis

Orthotopic & etastasis models:
Bio-imaging to monitor internal tumours & metastases.
- Evalution of tumours with Quantitative Bioluminescent Imaging:
A large panel of bioluminescent pre-clinical models which can be avaluated in real time using the optical molecular imaging system IVIS Spectrum from Caliper Life Sciences.
- Humanised Models:
- Tumour micro-environment imaging:
Dual bioluminescent/fluorescent biological reporters activated by different drugs or environmental stimuli
- Standard immunochemistry methods to evaluate:
- Proliferation, Apoptosis, Necrosis, Angiogenesis
- Diagnostic/biomarker detection
- ELISA analysis of serum and tissues for a comprehensive range of biological targets/marker
Vaccine Development and Immune Research
The research activity of the Vaccine Development and Immune Research laboratory team, is focused on developing tools for vaccine development and immune response monitoring, including antigen production, immunoenzymatic assay set-up and execution, polyclonal and monoclonal antibody production, and antibody characterization, and, as well.
Main Technologies
Carebios has at is disposal multiple host strains and cell lines, as well as a large library of IP-free expression vectors for the production of recombinant proteins and antibodies.
PF performance is ensured by the design of project-specific and tailored strategies and by the use of cutting-edge tools and methods. In addition, advanced analytical techniques, such as SPR, HPLC and LC-MS/MS, together with the standard biochemical analyses, are available for proteins and antibodies characterization.
Main activities
Recombinant Proteins production and characterization: Gene synthesis (with or without codon optimization) and expression plasmid preparation; Expression testing in the selected host (E. coli, P. pastoris, or Mammalian cells); Expression optimization and set-up of the purification method; Scale-up of protein expression and purification; Analysis of quantity, purity and integrity (UV-Vis, SDS-PAGE, HPLC, and Western Blot); Characterization (HPLC, LC-MS/MS), binding and kinetics studies (SPR); Endotoxin testing and removal; Protein and Antibody Labeling
Antibodies development: Immunization strategy design; Antigen design and production (optional); Immunization (Mice, Rats, Rabbits) and serum antibody titration; Hybridoma generation and screening (for Monoclonal Ab production); Stabilization and banking of the monoclonal culture; Purification of the antibodies (0.1-100 mg); Ab characterization (isotyping, SDS-PAGE, ELISA, HPLC, SPR)
Immunoassay development: Antibody selection and pairing; Generation of calibration curves for quantitative applications; Assay optimization, qualification, and analytical validation; Manufacturing and delivery of the assay kit; Fit-for-purpose ELISA modification of existing assays